The sum of the oxidation states within a compound or ion must equal the overall charge. Californium is one of the few transuranium elements that have practical applications.

Californium The Cambridge Crystallographic Data Centre Ccdc
The oxidation state of an atom is a measure of the degree of oxidation of an atom.
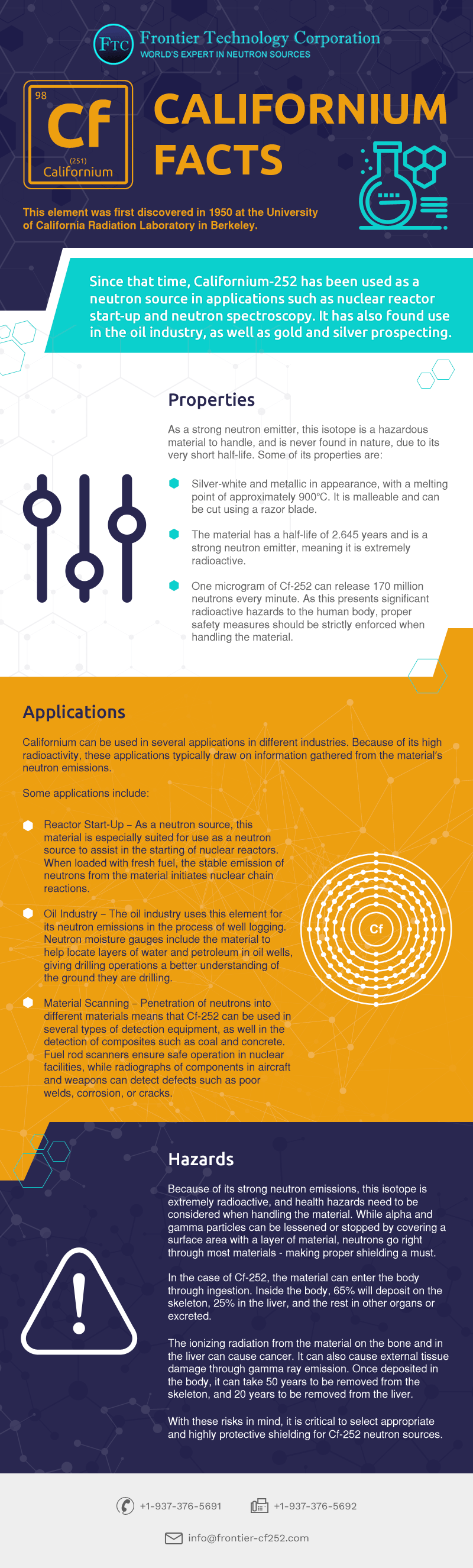
What are two common uses of californium. Sodium is a chemical element with the symbol Na from Latin natrium and atomic number 11. Its only stable isotope is 23 Na. It is a soft silvery-white highly reactive metalSodium is an alkali metal being in group 1 of the periodic table.
It is defined as being the charge that an atom would have if all bonds were ionic. Californium-252 with a half-life of about 2645 years is the most common isotope used and is produced at the Oak Ridge National Laboratory in the United States and the Research Institute of Atomic Reactors in Russia. Rhenium sits two places below manganese in the periodic table and its existence was first predicted by Mendeleev when he first proposed his periodic table in 1869.
The free metal does not occur in nature and must be prepared from compounds. In fact this group is unusual in that when the periodic table was first published it possessed only one known element manganese with at least two gaps below it. Uncombined elements have an oxidation state of 0.

Californium Everything You Need To Know With Photos Videos

Californium Podcast Chemistry World

Californium Cf Properties Health Effects Californium Uses
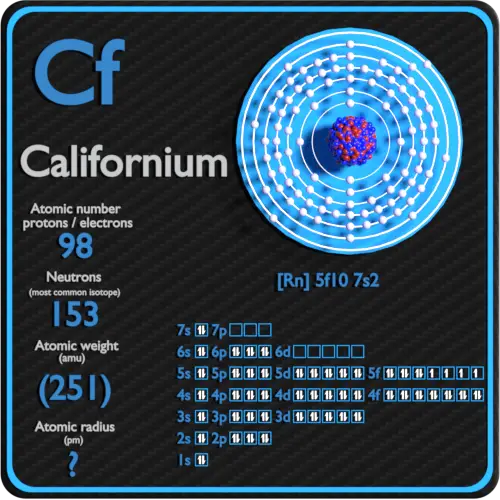
Californium Protons Neutrons Electrons Electron Configuration
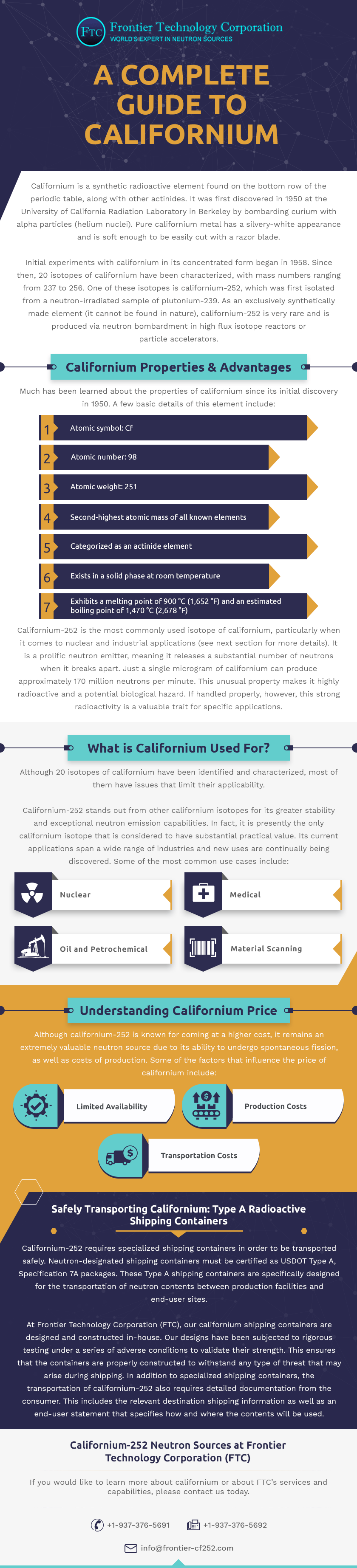
A Complete Guide To Californium Frontier Technology

Askus Why Is Californium Priced So High And What Exactly Does It Do For Us Kickassfacts Com

Californium Lesson For Kids Discovery Properties Study Com
